Wiley Series in Probability and Statistics - 2011 - Wiley Series in Probability and Statistics - Wiley Online Library

The Evolution of Master Protocol Clinical Trial Designs: A Systematic Literature Review - Clinical Therapeutics

Bayesian statistics and clinical trial designs for human cells and tissue products for regulatory approval - ScienceDirect
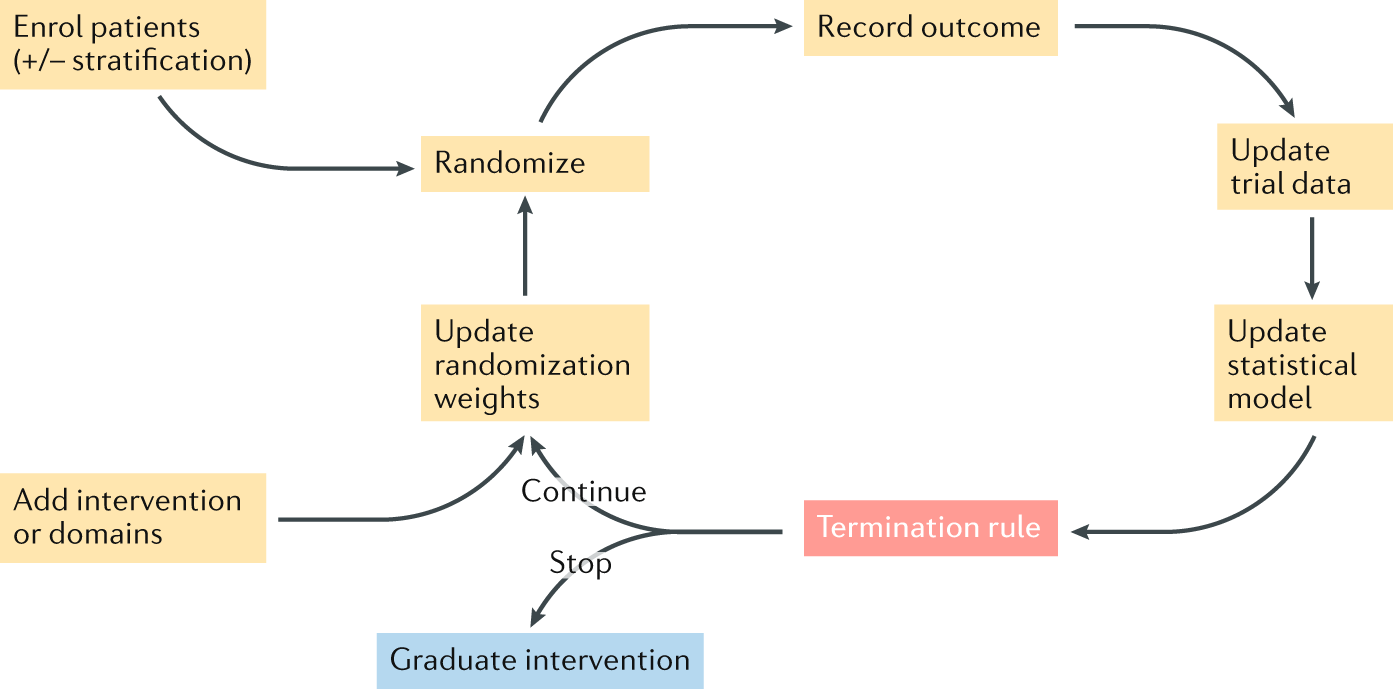
Adaptive platform trials: definition, design, conduct and reporting considerations | Nature Reviews Drug Discovery
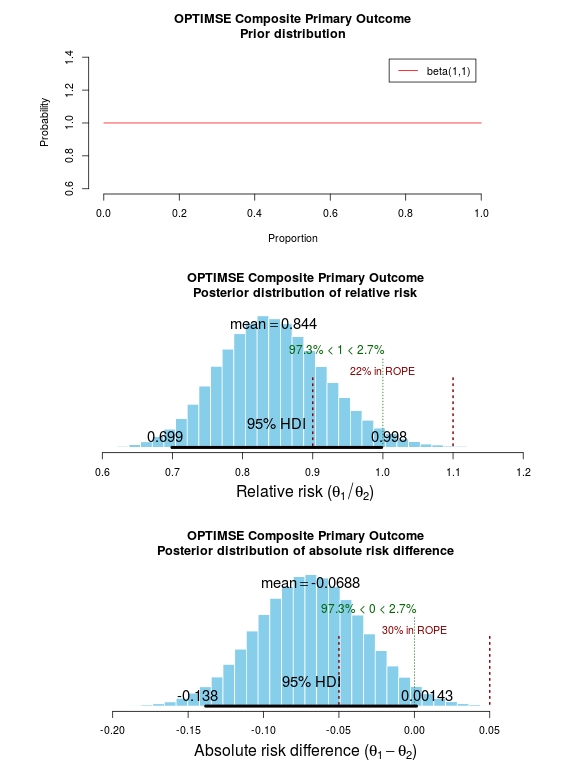
Bayesian statistics and clinical trial conclusions: Why the OPTIMSE study should be considered positive | R-bloggers
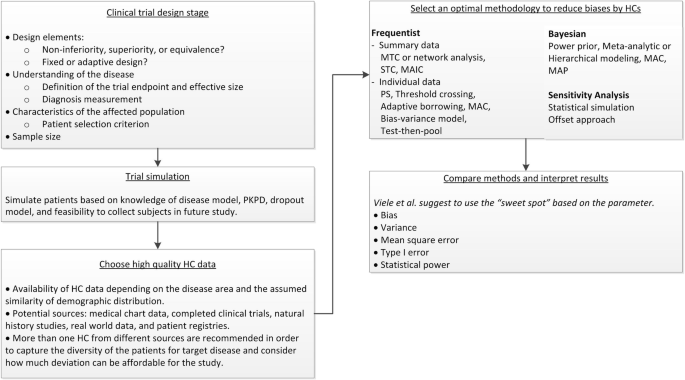
A roadmap to using historical controls in clinical trials – by Drug Information Association Adaptive Design Scientific Working Group (DIA-ADSWG) | Orphanet Journal of Rare Diseases | Full Text

Clinical Trial Design: Bayesian and Frequentist Adaptive Methods - Kindle edition by Yin, Guosheng. Professional & Technical Kindle eBooks @ Amazon.com.
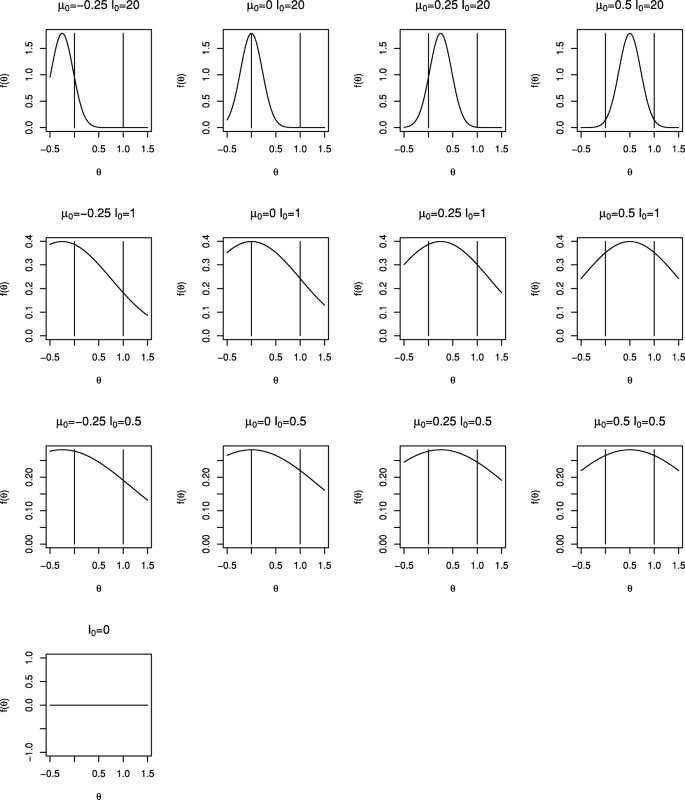
Comparison of Bayesian and frequentist group-sequential clinical trial designs | BMC Medical Research Methodology | Full Text
Ingram Olkin Forum Deliberates Bayes and Frequentist Approaches to Rescuing Disrupted Trials | National Institute of Statistical Sciences
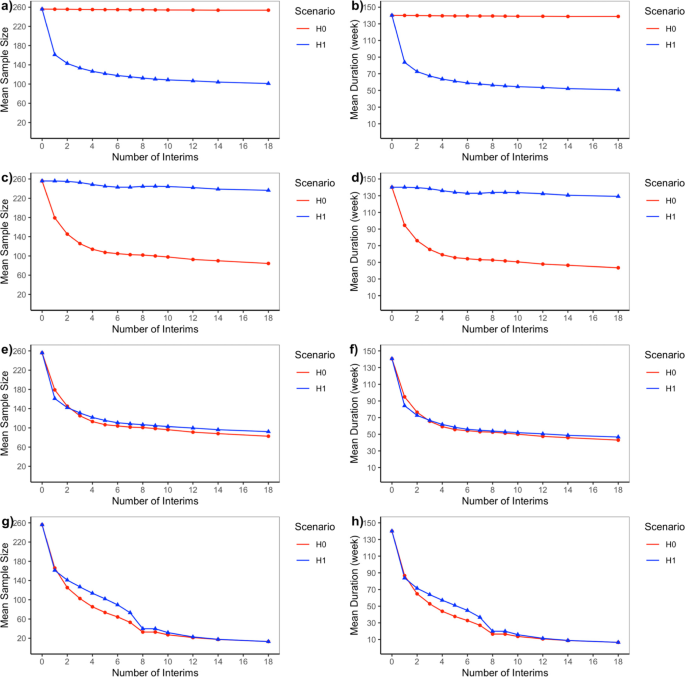
Bayesian adaptive design for pediatric clinical trials incorporating a community of prior beliefs | BMC Medical Research Methodology | Full Text








![PDF] Bayesian Adaptive Methods for Clinical Trials | Semantic Scholar PDF] Bayesian Adaptive Methods for Clinical Trials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f71d1b9c95e95f3a11a9cb77f479f1f5ea1df605/30-Figure2-1.png)