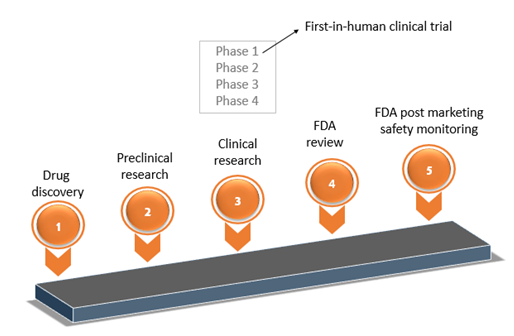
Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library

Design and Conduct Considerations for First‐in‐Human Trials - Shen - 2019 - Clinical and Translational Science - Wiley Online Library
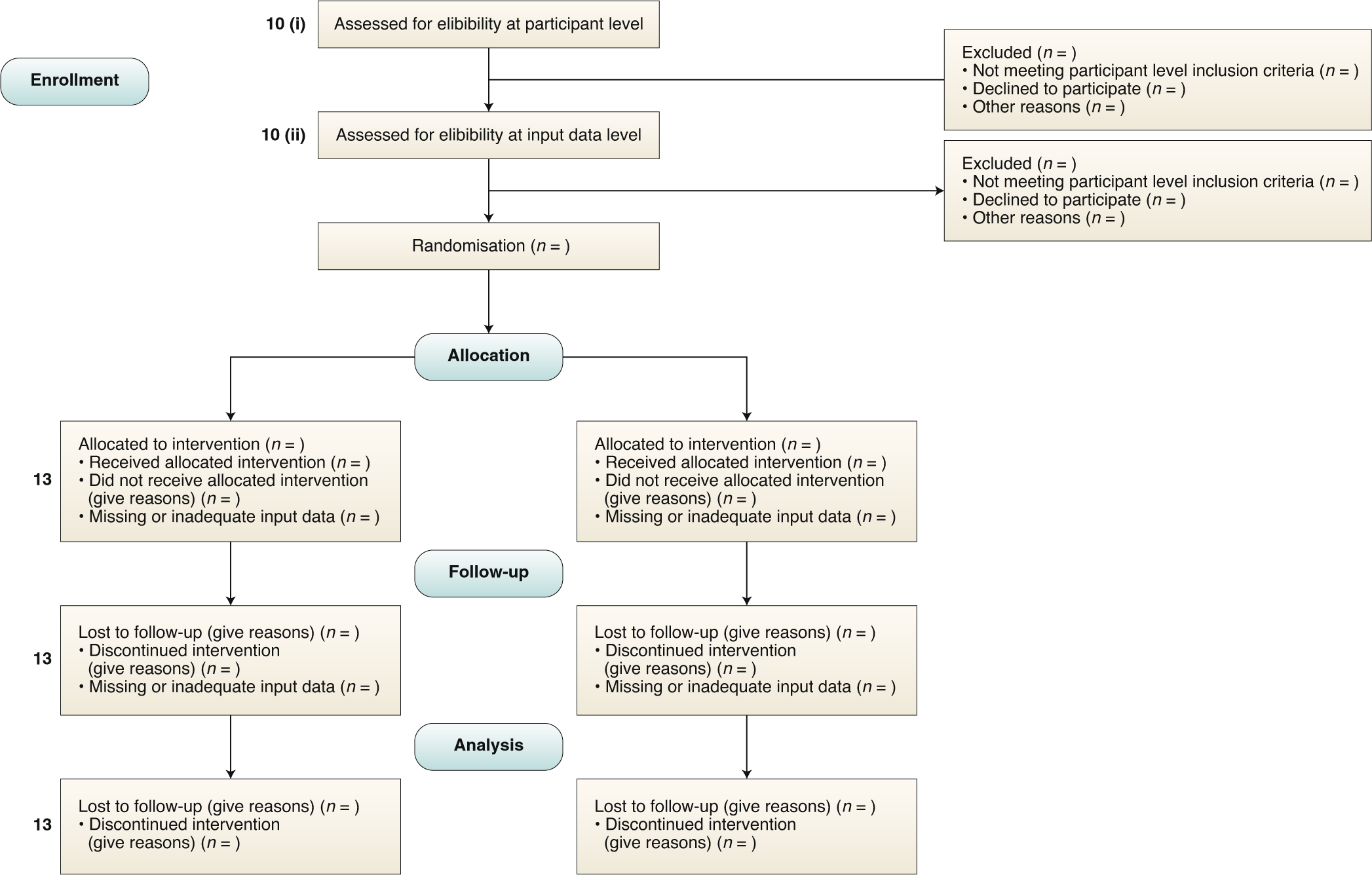
Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension | Nature Medicine

EMA publishes revised guidelines to reduce risk in first-in-human clinical trials - The Pharmaceutical Journal

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet