PPT - Robert O'Neill , Ph.D. Director, Office of Biostatistics, CDER, FDA PowerPoint Presentation - ID:7035315

PDF) ICH guideline “Statistical Principles for Clinical Trials”: issues in applying the guideline in practice

Early phase clinical trials extension to guidelines for the content of statistical analysis plans | The BMJ
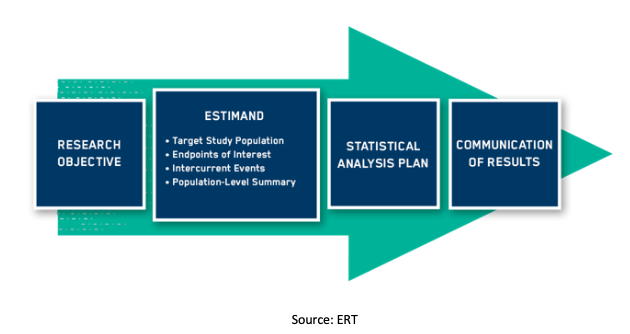
What is an estimand & how does it relate to quantifying the effect of treatment on patient-reported quality of life outcomes in clinical trials? | Journal of Patient-Reported Outcomes | Full Text

TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May PDF Free Download

The GetReal Trial Tool: design, assess and discuss clinical drug trials in light of Real World Evidence generation - Journal of Clinical Epidemiology
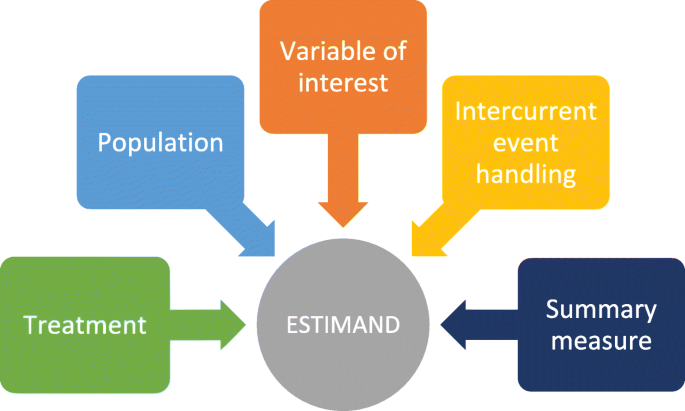
What is an estimand & how does it relate to quantifying the effect of treatment on patient-reported quality of life outcomes in clinical trials? | Journal of Patient-Reported Outcomes | Full Text

Tim Morris on Twitter: "The final 'ICH E9(R1) Addendum on estimands and senstivity analysis in clinical trials' was released on 3Dec2019. https://t.co/xwoh3QdV9Z https://t.co/UTV2w99amT" / Twitter

Estimating and reporting treatment effects in clinical trials for weight management: using estimands to interpret effects of intercurrent events and missing data | International Journal of Obesity

Statistics in Harmony: The Role of Estimands in Regulatory Writing - IMPACT Pharmaceutical Services, Inc.

ICH E9 guideline 'Statistical principles for clinical trials': a case study Response to A. Phillips and V. Haudiquet - Brown - 2003 - Statistics in Medicine - Wiley Online Library